 |
|
Femtosecond Laser Mass Spectrometry
Mass spectrometry is a highly sensitive and selective technique
suited to the analysis of organic as well as many other complex molecules.
In general, mass spectrometers operate on the principle of converting
neutral species (atoms or molecules) into gaseous ions and subsequently
separating those ions according to their mass-to-charge (m/z) ratio.
The highly selective and sensitive analytic technique of femtosecond
laser mass spectrometry (FLMS), is well suited to analytical studies
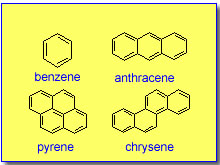
of organic molecules such as polycyclic aromatic hydrocarbons (PAHs)
and their nitro derivatives. These molecules are fused aromatic rings
and are environmental pollutants, with the highly polar nitroo-derivatives
considered to be significantly more carcinogenic.

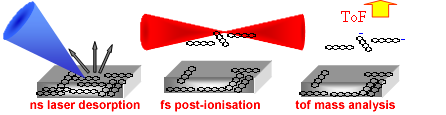
The evolution of FLMS as an analytical technique requires the use of methods to introduce solid-phase species to the gas-phase for ionisation. In addition to studies of elemental and inorganic materials, the potential of FLMS for the analysis of biomolecules and the detection of environmentally-hazardous materials is considerable. Recent studies have focused on coupling the TOPS femtosecond laser (800 nm, 50 fs, Intensity ~ 1015 W/cm2) with a laser desorption source (266 nm, 16 ns) for the analysis of solid-phase labile molecules. A technique kown as Laser Desorption/Femtosecond Laser Mass Spectrometry (LD/FLMS).
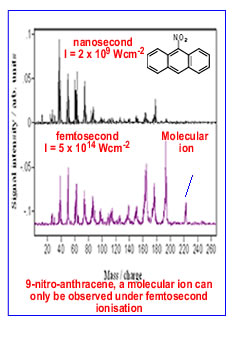
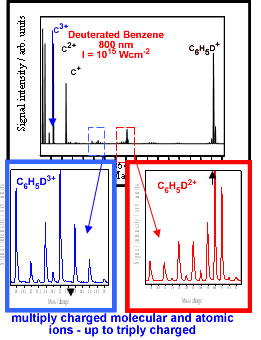
In analytical terms, an essential feature of mass spectrometry
is the unambiguous detection of ion signals (molecular ions). In the
case of nitro-PAHs, pulses of nanosecond duration are unable to analyse
the molecules due to their rapid dissociation prior to detection. On
the other hand, pulses of femtosecond duration have been shown to ionise
virtually all classes of molecules, almost eliminating the chance of
the molecule dissociating before it is detected. In additon, at high
intensities, multiply charged atomic ions (from coulomb explosions)
and molecular are observed. Examples of the above phenomena are shown
below.
|
Part of the
Scottish Universities
Physics Alliance (SUPA) and the
Department
of Physics, |
